Regenerative Orthopedics

Research lines
The Tryfonidou group focusses on three research lines:
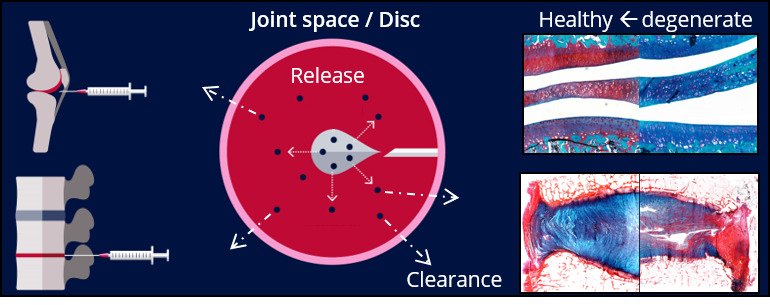
1. Local controlled drug delivery for prolonged pain relief in joints
Upon an intra-articular or intra-discal application, biomaterials enabling controlled release of anti-inflammatory medication harness inflammation and mitigate pain. Through a unique preclinical platform employing small animal models and ultimately the veterinary patients suffering from osteoarthritis or back pain, we have been able to develop these strategies.
To feed these strategies with the appropriate biomolecules, we employ developmental biology and the divergence seen within the canine species to identify and validate regenerative targets by means of “omics”. Within this context, we also study how joint distraction facilitates intrinsic cartilage repair.
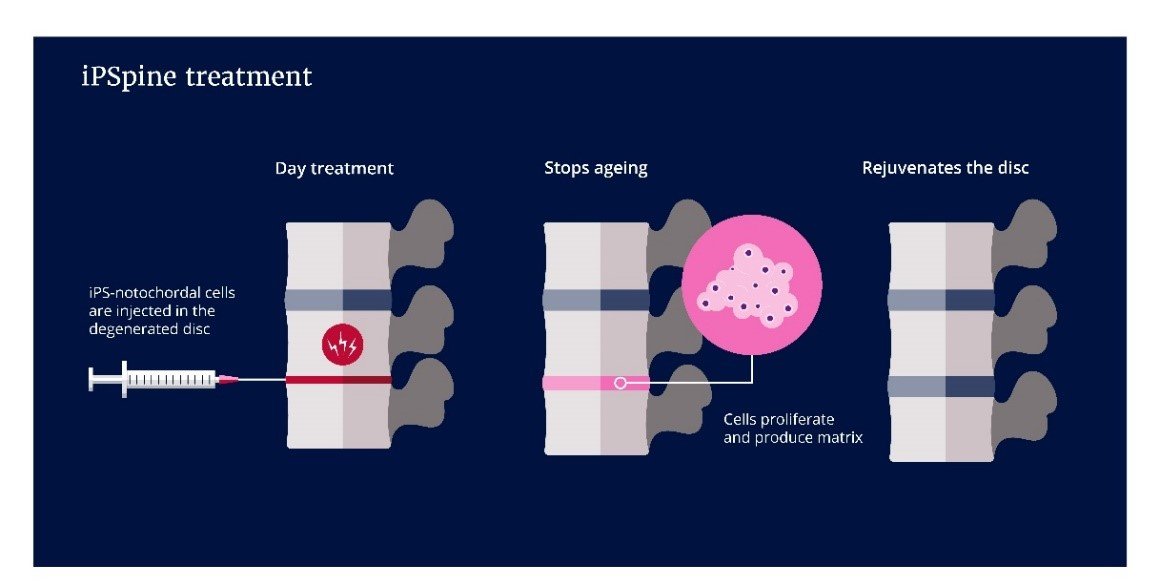
2. Cell-based therapies to regenerate intervertebral discs and reverse disease
Long-lasting reduction of back pain can only be achieved via regeneration of tissue and restoration of the biomechanical function of the disc. However, stem cell-therapies exert short-lived analgesic effects and do not regenerate the disc. In this research line, we employ notochordal cell biology and their regenerative and anti-inflammatory potential.
Within the H2020 iPSpine consortium, we focus on differentiating induced pluripotent stem cells (iPS) into notochordal cells, the cells that reside in juvenile discs. These iPS-NCs, combined with smart biomaterial, are geared to rejuvenate the degenerate disc. Initial studies in preclinical testing platforms will culminate in a proof-of-concept study in canine patients suffering from chronic back pain.
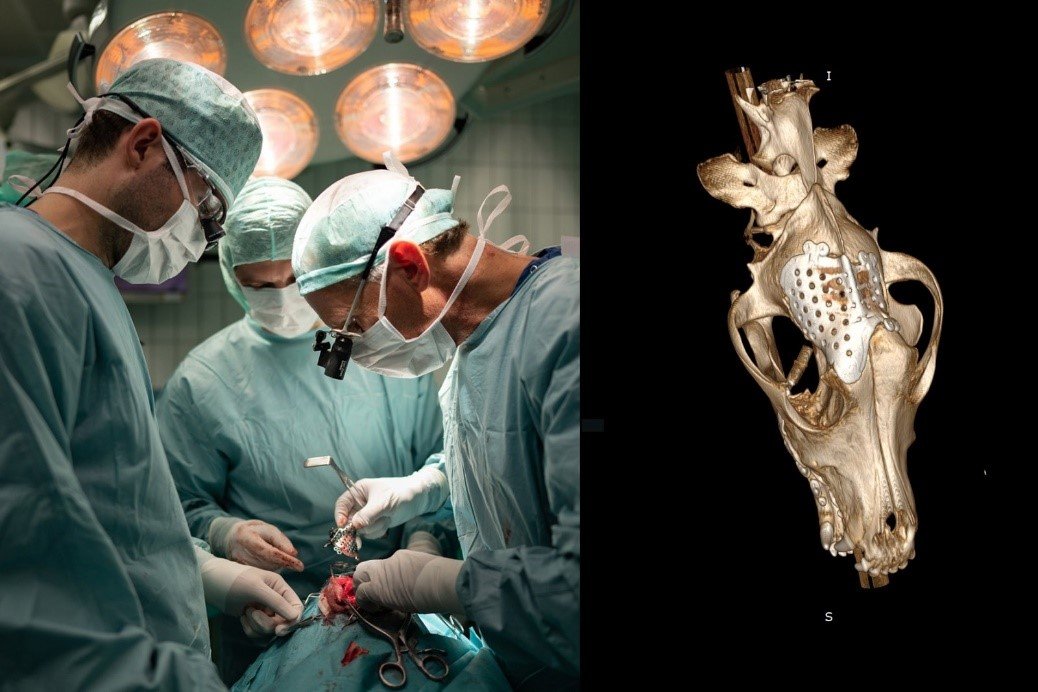
3. Personalized 3D-printed implants.
In collaboration with the University Medical Centre and the Field lab 3DMedical we implement personalized implants to treat complicated veterinary orthopaedic cases. In addition, we are currently further developing a safe and straightforward surgical approach to treat hip dysplasia in human and veterinary patients with a personalized 3D-printed titanium implant. This treatment should prevent the development of debilitating secondary hip osteoarthritis and prevent last resort surgical treatment in severe cases involving femoral head and neck resection or hip joint replacement.
People
Name | Position | Contact/Linkedin | Additional info |
|---|---|---|---|
| Marianna Tryfonidou | Professor | m.a.tryfonfidou[at]uu.nl | Principal Investigator, group leader. |
| Björn Meij | Professor | b.p.Meij[at]uu.nl | Veterinary orthopedics surgeon. Implement personalized 3D-printed implants for the treatment of hip dysplasia. |
| Frances Bach | Postdoc | f.c.bach[at]uu.nl | IVD regeneration strategies with extracellular vesicles derived from notochordal cells, large animal models (H2020 iPSpine project). |
| Deepani Poramba-Liyanage | Postdoc | d.w.l.porambaliyanage[at]uu.nl | epigenetics and iPSC differentiation, single cell sequencing of disc cells (H2020 iPSpine project). |
| Lizette Utomo | Scientific Project & Research manager | l.utomo[at]uu.nl | Project manager for H2020 iPSpine and scientifically involved in the research line: “local controlled drug delivery”. |
| Joost von Hegedus | Postdoc | j.h.vonhegedus[at]uu.nl | New Therapeutic Targets for Osteoarthritis, Equine Musculoskeletal Biology, Lipid Signaling, Cell Biology and Immunology. Involved in William Hunter, Jointsphere, Regmed, Eurostar projects. |
| Amir Kamali | Postdoc | A.S.Kamali[at]uu.nl | Biomimetic disc prosthesis in collaboration with TU/e, BioAID Project |
| Michelle Teunissen | PhD candidate | m.teunissen[at]uu.nl | chondrogenesis & osteoarthritis, knee joint distraction, canine models (NWO Perspectief; William Hunter Revisited project). |
| Lisanne Laagland | PhD candidate | l.t.laagland[at]uu.nl | IVD regeneration strategies with iPSCs and notochordal cells (H2020 iPSpine project). |
| Josette van Maanen | PhD candidate | j.c.vanmaanen[at]uu.nl | Regenerative and anti-inflammatory effects of extracellular vesicles derived from notochordal cells (H2020 iPSpine project). |
| Xiaole Tong | PhD candidate | x.tong[at]uu.nl | Epigenetics and iPSC differentiation for disc regeneration, iPS-based reporter lines (H2020 iPSpine project). |
| Frank Riemers | Bioinformatician | F.M.Riemers[at]uu.nl | Expertise in bioinformatics, R programming, single cell RNAseq, and qPCR. |
| Saskia Plomp | Senior technician | S.G.M.Plomp[at]uu.nl | Expertise in cell culture, histology, immunohistochemistry, biochemical assays |
| Adel Medzikovic | Technician | a.medzikovic[at]uu.nl | Expertise in iPS and notochordal cell culture, qPCR, immunofluorescence |
| Lianne Snel | Technician | l.snel[at]uu.nl | Expertise in biomolecular research, iPS cell culture, histology |
Publications
| "Old Drugs, New Tricks" - Local controlled drug release systems for treatment of degenerative joint disease. Tryfonidou MA, de Vries G, Hennink WE, Creemers LB. Adv Drug Deliv Rev. 2020 Oct 27;160:170-185. |
| Notochordal-cell derived extracellular vesicles exert regenerative effects on canine and human nucleus pulposus cells. Bach F, Libregts S, Creemers L, Meij B, Ito K, Wauben M, Tryfonidou M. Oncotarget. 2017 Oct 4;8(51):88845-88856. doi: 10.18632/oncotarget.21483. |
| Tellegen AR, Dessing AJ, Houben K, Riemers FM, Creemers LB, Mastbergen SC, et al. Dog as a Model for Osteoarthritis: The FGF4 Retrogene Insertion May Matter. J Orthop Res. 2019;37(12):2550-60. |
| Rudnik-Jansen I, Tellegen AR, Pouran B, Schrijver K, Meij BP, Emans PJ, et al. Local controlled release of corticosteroids extends surgically induced joint instability by inhibiting tissue healing. Br J Pharmacol. 2019;176(20):4050-64. |
| Tellegen AR, Rudnik-Jansen I, Beukers M, Miranda-Bedate A, Bach FC, de Jong W, et al. Intradiscal delivery of celecoxib-loaded microspheres restores intervertebral disc integrity in a preclinical canine model. J Control Release. 2018;286:439-50. |
| Bach FC, Tellegen AR, Beukers M, Miranda-Bedate A, Teunissen M, de Jong WAM, et al. Biologic canine and human intervertebral disc repair by notochordal cell-derived matrix: from bench towards bedside. Oncotarget. 2018;9(41):26507-26. |
| Tellegen AR, Rudnik-Jansen I, Pouran B, de Visser HM, Weinans HH, Thomas RE, et al. Controlled release of celecoxib inhibits inflammation, bone cysts and osteophyte formation in a preclinical model of osteoarthritis. Drug Deliv. 2018;25(1):1438-47. |
| Krouwels A, Melchels FPW, van Rijen MHP, Ten Brink CBM, Dhert WJA, Cumhur Oner F, et al. Focal adhesion signaling affects regeneration by human nucleus pulposus cells in collagen- but not carbohydrate-based hydrogels. Acta Biomater. 2018;66:238-47. |
| Teunissen M, Riemers FM, van Leenen D, Groot Koerkamp MJA, Meij BP, Alblas J, et al. Growth plate expression profiling: Large and small breed dogs provide new insights in endochondral bone formation. J Orthop Res. 2018;36(1):138-48. |
Alumni
Name | Contact/Linkedin | Additional info |
|---|---|---|
| Maurits Olthof | https://www.linkedin.com/in/maurits-olthof-b252811aa/ | PhD Thesis Defended: 2019 |
| Imke Rudnik-Jansen | https://www.linkedin.com/in/imke-rudnik-jansen-202567163/ | PhD Thesis Defended: 2019 |
| Anna Tellegen | https://www.linkedin.com/in/anna-tellegen-b8675245/ | PhD Thesis Defended: 2019 |
| Anita Krouwels | https://www.linkedin.com/in/anitakrouwels/ | PhD Thesis Defended: 2019 |
| Luc Smolders | https://www.linkedin.com/in/luc-smolders-38430834/ | PhD Thesis Defended: 2013 |
Contact for internships
Prof. Dr. Marianna Tryfonidou, Principal investigator, m.a.tryfonfidou[at]uu.nl

