Enzyme-mystery solved after 40 years
Publication in Science
Scientists have been searching for it for decades: the enzyme that cuts the amino acid tyrosine off an important part of the cell's skeleton. Researchers of the Netherlands Cancer Institute, the Hubrecht Institute and Maarten Altelaar of Utrecht University, have now identified this mystery player, which may be of vital importance to the understanding of cell function and division, and therefore the understanding of cancer. They published their finding in Science on November 16th.
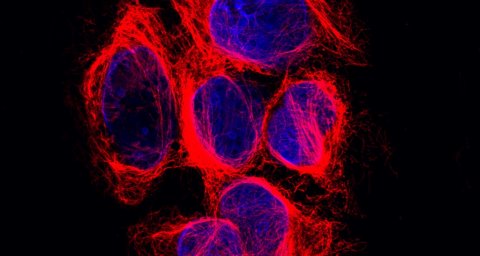
Just like the human body as a whole, each human cell has a skeleton it needs for functioning properly. That so-called cytoskeleton allows a cell to maintain its shape, move to different places and transport molecules through its interior. Long chains called microtubules (see image) form an important part of that skeleton and function as a highway for the transport of molecules. For example, microtubules play a key role in cell division by allowing the cell to meticulously align their chromosomes before dividing them amongst daughter cells. Their crucial importance to the cell is easily illustrated by the working mechanism of a widely used group of cancer medicines called taxanes: they disrupt microtubule function and thereby kill dividing cells.
Four decades
It has been suggested that proper transport at this crucial moment in the cell cycle involves detyrosination, in which the amino-acid tyrosine is removed from the tail of one of the microtubules' main building block: α-tubulin. Over the past four decades scientist have been searching for the main actor in this process. Despite its importance for several cellular processes it remained unknown which enzyme takes off the tyrosine.

Innovative method
Researchers of the Netherlands Cancer Institute, the Hubrecht Institute and Utrecht University, have now solved this puzzle. They managed to do this through an innovative genetic screening method published in Nature last May, that was developed by amongst others Maarten Altelaar and three other authors of the Science-publication. For this they used genome wide random mutations in human cells, containing just one copy of each gene. Then they selected the cells in which the studied process of detyrosination was broken, due to one of the randomly introduced mutations. Subsequently they analysed the cells with very little detyrosinated tubulin and discovered they had a mutated (and therefore dysfunctional) SVBP gene.
Surprising
This led them to identify the small SVBP protein as being a crucial part of the process. This small protein binds - and thereby stabilizes - proteins called vasohibins, which appear to have tubulin detyrosination activity. Nieuwenhuis: "These findings are surprising, because vasohibins were thought to function outside the cell and only recently it was predicted that these proteins might function as enzymes, without knowing their function."
Confirmation
Further experiments confirmed the interaction with vasohibins and its effect on tubulin detyrosination. For part of this confirmation, the characterization of the N-terminal tail of the protein, a method developed by the Biomolecular Mass Spectrometry and Proteomics group of Utrecht University was applied. This method was published in Nature Methods in 2008.
Important step
"For cell biologists this could be an important step", says Nieuwenhuis. "We have found a piece of the puzzle that scientist have been staring at for many years because the process of detyrosination was discovered 40 years ago. This knowledge could be relevant to further understand the processes of mitosis, cell migration and cancer development. It is already found that the invasive front in some tumour tissues, where cells are migrating most actively, contains a high amount of detyrosinated tubulin. It is interesting to speculate that inhibition of detyrosination could be beneficial under certain conditions."
Similar conclusions
Interestingly, in the same edition of Science a group of French scientists reached similar conclusions using a biochemical approach to identify detyrosinating enzymes.
Publicatie
Vasohibins encode tubulin detyrosinating activity
Joppe Nieuwenhuis, Athanassios Adamopoulos, Onno B. Bleijerveld, Abdelghani Mazouzi, Elmer Stickel, Patrick Celie, Maarten Altelaar, Puck Knipscheer, Anastassis Perrakis, Vincent A. Blomen, Thijn R. Brummelkamp
Science, 16 November 2017
This news item is a slightly adapted version of the press release of NKI.

