Molecular architecture of entry gate for human cell visualized
Publication in Nature
Scientists have clarified how an ion channel opens and closes in human cells. This channel, called a membrane protein, acts as a kind of gate to determine which ions are allowed to enter or leave the cell. Together with researchers from the Oregon Health & Science University and the University of Illinois, chemist and PhD student at Utrecht University Wout Oosterheert studied the molecular structure of the membrane protein, which may present a promising target for the development of medication. Their results were published last week in Nature.
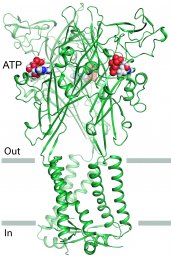
The membrane protein that Oosterheert focused on is the P2X3 protein. This receptor is present throughout the human body, including nerve cells and cells in the cardiovascular and immune systems. It acts as a channel through the cell membrane, transporting ions such as sodium and calcium in and out of the cell. A ‘gate’ in the protein determines whether the particles can be allowed in or out of the cell.
Opening and closing
The study that Oosterheert participated in has now shown exactly how the gate is opened from the resting position as adenosine triphosphate (ATP) binds to the receptor. It furthermore showed how the gate closes again using a different mechanism. The researchers have also determined how some substances can stabilise the resting state of P2X3, preventing the channel from opening. These inhibitors, or ‘antagonists’, act as a lock on the channel.
Unstable
In order to determine the structure of the receptor in various states - resting, open and closed - Oosterheert and his colleagues used X-ray crystallography. In this technique, crystals of the P2X3 protein are exposed to X-ray radiation, and the scientists can then examine the diffractionpatterns to view the structure of the crystal. “Studying membrane proteins like P2X3 is very difficult, because they are often unstable outside of their natural environment”, Oosterheert explains. “Plus, it wasn’t easy to isolate the protein in a specific state. So I developed a special method for purifying the P2X3 receptor, which made it possible to determine the molecular architecture in the resting state.” He conducted the research while he was working in the lab of Professor Eric Goaux at the Oregon Health & Science University in Portland for seven months.
Stimulus
Oosterheert expects that the results will be a stimulus for further pharmacological research. “We have now solved the gating cycle for this membrane protein. We know how the gate opens and closes again. This is vital for the fundamental understanding of these kinds of proteins.”
Publication
X-ray structures define human P2X3 receptor gating cycle and antagonist action.
Steven E. Mansoor,Wei Lü, Wout Oosterheert*,Mrinal Shekhar,Emad Tajkhorshid & Eric Gouaux
Nature (2016) doi:10.1038/nature19367
* affiliated with Utrecht University

