Dr. Toon de Kroon
dr. A.I.P.M. (Toon) de Kroon
Email: a.i.p.m.dekroon@uu.nlPhone: 030 253 3424Address
Padualaan 8
3584CH Utrecht
Membrane Lipid Homeostasis
Membrane lipid composition determines the physical properties of biological membranes that are crucial for maintaining the membrane barrier and for the functioning of membrane proteins. Research in the group is aimed at understanding the principles that govern bulk membrane lipid composition and at identifying the underlying sensor-effector modules. These topics are investigated in the model eukaryote S. cerevisiae using biochemical, molecular biological, genetic and chemical biological approaches.
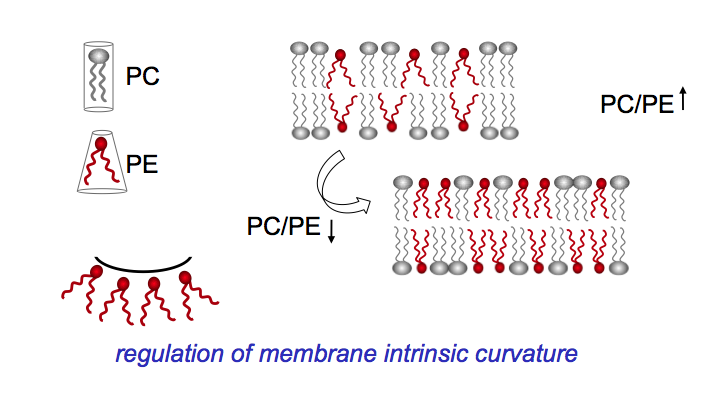
Interplay between membrane lipid class and acyl chain composition
By gradually reducing the content of phosphatidylcholine (PC) from 40 % to below 1% of total membrane phospholipids we identified a novel regulatory mechanism for membrane lipid composition in yeast. The loss of the bilayer lipid PC is compensated for by a rise in the non-bilayer preferring lipid phosphatidylethanolamine (PE). Mass spec analysis revealed that PC depletion is accompanied by dramatic changes in the acyl chain composition of PE that reduce its non-bilayer propensity.
Thus, membrane fluidity and intrinsic membrane curvature are maintained in the optimal range. The current challenge in the project of Xue Bao is to elucidate the underlying sensing mechanism and regulatory network. The isolation of PC-free yeast suppressor mutants provides an important new tool in this research.
Metabolism of phospholipids at the molecular species level
Pulse labeling with stable isotope-labeled precursors and subsequent analysis of phospholipid species by ESI-MS/MS demonstrated the occurrence of PC remodeling by acyl chain exchange. We now want to identify the phospholipases and acyltransferases involved, with the ultimate aim of solving the function of PC remodeling. Manipulation of the acyl chain composition of yeast by overexpression of the G-3-P acyltransferase Sct1 is an important tool in these projects.

