Why is iron magnetic, unlike other metals?
Many materials become magnetized when you hold them close to a magnet. Only a few elements can hold on to this magnetization.
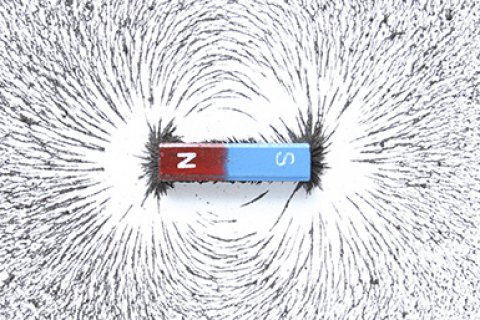
Remanent magnetization
Many materials become magnetized when you hold them close to a magnet. In other words, they behave in a 'magnetic' way. Only a few elements, including iron, can hold on to this magnetization when the magnet is removed. Such a magnetization is called a spontaneous or remanent magnetization.
Nickel, cobalt and gadolinium
Iron is not the only material capable of possessing a remanent magnetization. Nickel, cobalt and the lesser known gadolinium can do that as well, along with compounds that contain one of these four metals. Who really wants to understand this phenomenon will quickly stumble upon quantum mechanics: the complex science on the behaviour of matter and energy on a very small, (sub)atomic scale.
Spins
The occurrence of magnetization is explained by means of 'spins': a quantum mechanical concept that can describe the energy management of electrons. Exactly in the four elements iron, nickel, cobalt and gadolinium, there is interaction between so-called 'unpaired spins'. This interaction ensures that the magnetic moments of atoms can permanently align parallel to each other. The sum of all these small magnetizations forms the nett magnetization of the material.
The most powerful remanent magnets are made of neodymium
The most powerful remanent magnets are made of chemical compounds that contain rare earth minerals, such as neodymium. In magnets like those, the atoms are arranged in such a way that all the unpaired spins are forced in the same direction to form a very powerful magnetic moment.

