Simulations point the way to cheaper production of quantum dots
"We now can finally understand the process of quantum dots formation"
The production of quantum dots for uses such as better displays for televisions and smartphones may be possible for a much lower price. That is the result of computer simulations of the formation of quantum dots, for which researchers at Utrecht University and TU Delft developed a model. These simulations can also be used to design new types of nanoparticles with interesting functional characteristics. The research, which is led by Dr. Marijn van Huis (Utrecht University) and Prof. Thijs Vlugt (TU Delft) was published today in Nature Communications.
According to our current understanding, the formation of quantum dots can take several hours. In principle, these types of processes occur faster at higher temperatures, but in the case of quantum dots this would make the system unstable. “But thanks to our simulations, we can finally understand the process of quantum dot formation. That makes it easier to optimise the process. Moreover, we expect that this will eventually enable us to design and produce entirely new types of functional quantum dots and other nanoparticles with interesting characteristics”, according to Thijs Vlugt, Professor of Engineering Thermodynamics at TU Delft.
Ion exchange
Quantum dots are created in a solution. An important element of the process of synthesizing quantum dots is the replacement of one type of atom with another. In this study, lead atoms were replaced by cadmium atoms to help facilitate ion exchange. But until know, scientists were uncertain how this ion exchange occurred. They were unable to simulate the process, because the atomic system is too large and complicated. However, the researchers from Delft and Utrecht have developed a model that is good enough to enable them to conduct simulations.
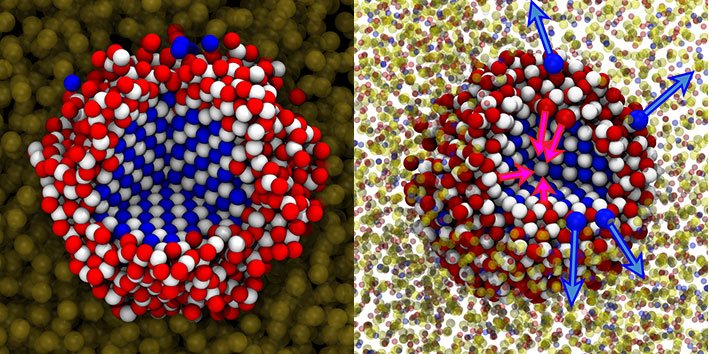

These simulations show that quantum dots form much faster and with a much higher yield by conducting the process at a higher pressure as well as a higher temperature. “In several cases, this can even reduce the production time from hours to just minutes”, explains Dr. Marijn van Huis from Utrecht University. “There are already some televisions on the market that use screens made with quantum dots. Screens with quantum dots are much clearer, have a wider colour range and are more energy-efficient. Large-scale production is currently too expensive to be profitable, but that may change very soon.”
Much too complex
The main problem for the simulations were the ligands, long molecules in the solution that performs the ion exchange. These ligands concentrate around the quantum dot, transporting one ion from the solution to the quantum dot and the other back to the solution after they are exchanged. The entire interaction between the quantum dot, the ligands and the solution is much too complex to simulate as an atomic system. In their publication, Van Huis, Vlugt and their colleagues show that it is possible to simplify this system by considering the ligand and the solution as a single element of the simulation.
Experimental research
The researchers tested their model with simulations of the formation of a certain type of nanoparticles that has already been the subject of much experimental research. The results of these simulations closely corresponded to the results of the experiments.
This research was funded by Stichting FOM.
Publication
‘Atomistic understanding of cation exchange in PbS nanocrystals using simulations with pseudoligands’
Zhaochuan Fan, Li-Chiang Lin, Wim Buijs, Thijs J.H. Vlugt & Marijn A. van Huis
Nature Communications, 10 mei, DOI 10.1038/NCOMMS11503.
ERC Consolidator Grant
Dr. Marijn van Huis recently received a ERC Consolidator Grant, read the news release.

